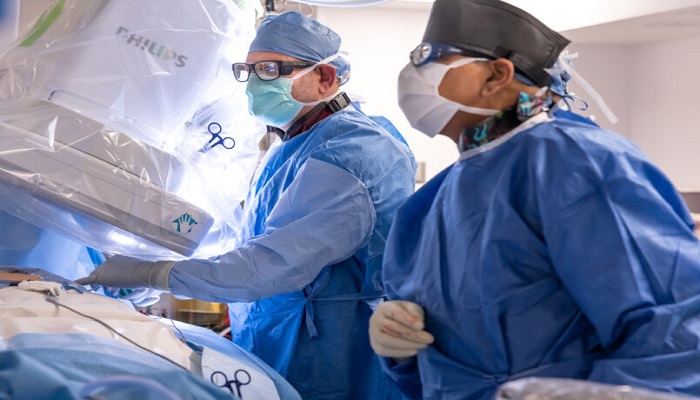
Cleveland Clinic has successfully implanted a dual cardiac device in the first patient in the world as part of a clinical trial, which aims to potentially treat heart failure symptoms.
The INTEGRA-D clinical study will evaluate the safety and effectiveness of a device that combines two proven cardiac therapies into one. Cardiac contractility modulation works to improve the contraction of the heart, while an implantable cardioverter defibrillator (ICD) treats life-threatening arrhythmias that cause sudden cardiac death.
“This could be an important advancement for heart failure patients, requiring just one procedure to deliver two important therapies and prevent sudden cardiac death,” said Bruce Wilkoff, M.D., director of Cardiac Pacing and Tachyarrhythmia Devices at Cleveland Clinic and principal investigator for the INTEGRA-D study. “The hope is that this rechargeable technology – with a potential battery life of up to 20 years – will significantly reduce the need for replacement procedures.”
According to the Centers for Disease Control and Prevention, heart failure affects an estimated 6.2 million Americans. These patients experience debilitating symptoms, including breathlessness, fatigue, confusion and swelling in the legs that can greatly diminish quality of life. Most heart failure patients are prescribed medications that work to slow the disease’s progression and manage symptoms, but effectiveness can wane over time. While standard, existing implantable cardiovascular defibrillators are lifesaving, the technology alone does not improve the debilitating symptoms of heart failure.
“We are looking forward to studying this new technology to determine its potential to advance treatments for patients living with heart failure,” said Niraj Varma, M.D., Ph.D., professor of medicine, Cleveland Clinic Lerner College of Medicine, consultant electrophysiologist at Cleveland Clinic London and national primary investigator of the INTEGRA-D clinical trial.
The clinical trial, sponsored by Impulse Dynamics, manufacturer of the device, will enroll 300 patients from 75 centers across the U.S. who will be followed for two years.
About Cleveland Clinic
Cleveland Clinic is a nonprofit multispecialty academic medical center that integrates clinical and hospital care with research and education. Located in Cleveland, Ohio, it was founded in 1921 by four renowned physicians with a vision of providing outstanding patient care based upon the principles of cooperation, compassion and innovation. Cleveland Clinic has pioneered many medical breakthroughs, including coronary artery bypass surgery and the first face transplant in the United States. U.S. News & World Report consistently names Cleveland Clinic as one of the nation’s best hospitals in its annual “America’s Best Hospitals” survey. Among Cleveland Clinic’s 77,000 employees worldwide are more than 5,658 salaried physicians and researchers, and 19,000 registered nurses and advanced practice providers, representing 140 medical specialties and subspecialties. Cleveland Clinic is a 6,665-bed health system that includes a 173-acre main campus near downtown Cleveland, 22 hospitals, more than 275 outpatient facilities, including locations in northeast Ohio; southeast Florida; Las Vegas, Nevada; Toronto, Canada; Abu Dhabi, UAE; and London, England. In 2022, there were 12.8 million outpatient encounters, 303,000 hospital admissions and observations, and 270,000 surgeries and procedures throughout Cleveland Clinic’s health system. Patients came for treatment from every state and 185 countries.
Source: Cleveland Clinic